
Secondly, the p-i model proposes that small molecule drugs may bind non-covalently to HLA or T cell receptors and directly stimulate T cells ( 5). Furthermore, the sites where drug modification occurs have been mapped using mass spectrometry and synthetic penicilloyl-adduct peptides have been shown to be more potent stimulators of T cells in patients with penicillin hypersensitivity ( 6). Beta-lactam antibiotics have been shown to behave in this manner, as drug modified human serum albumin has been isolated from individuals utilizing piperacillin, penicillin-G and flucloxacillin ( 6).
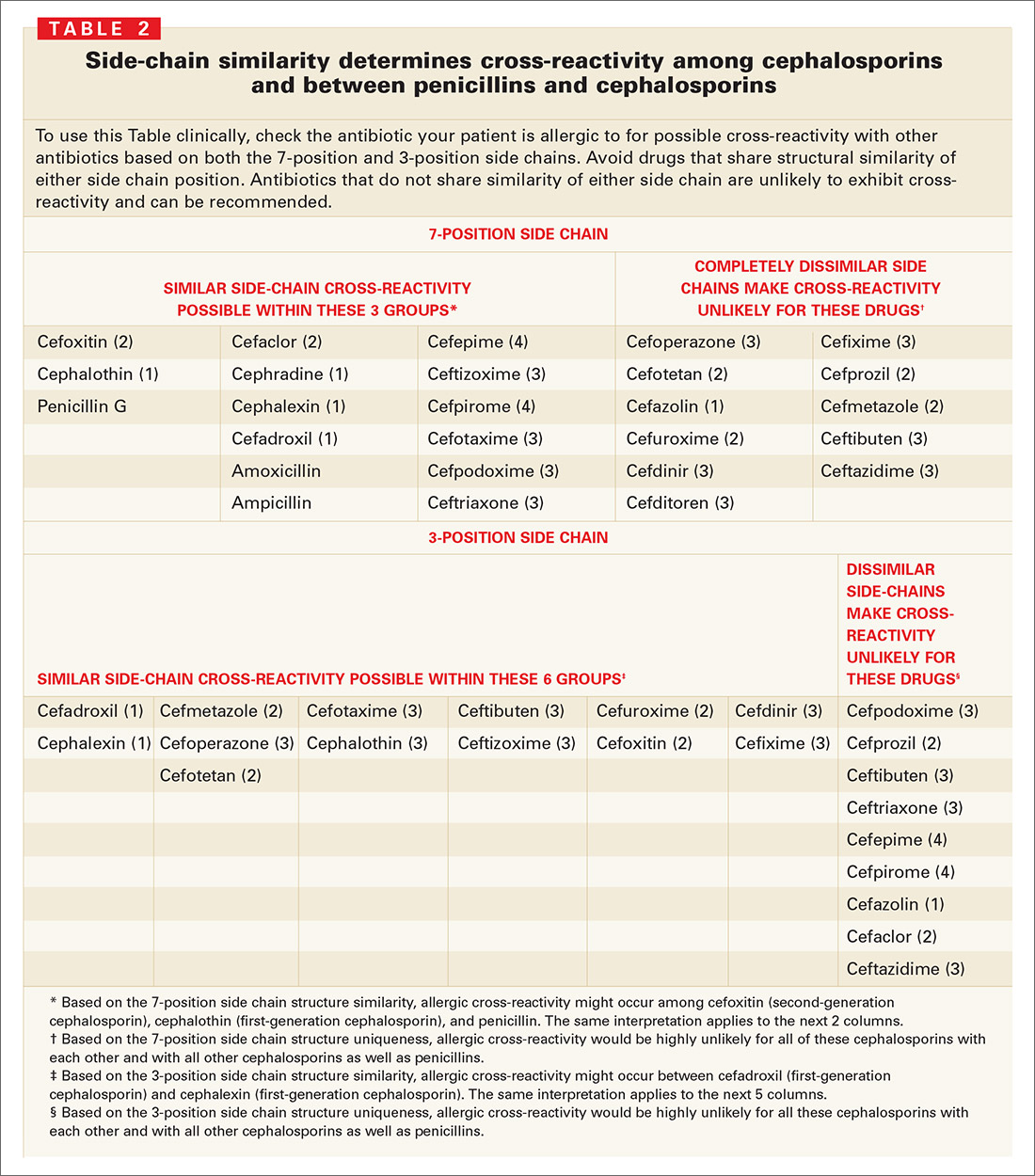

Firstly, in the hapten/prohapten model a drug binds to a protein that then undergoes antigen processing to generate haptenated-peptides which are recognized as neo-antigens by T cells. The interactions between the TCR, HLA molecule and the offending drug are thought to occur in three possible ways. Interactions between the culprit drug, human leukocyte antigen (HLA) class I molecules and T cell receptors (TCR) in addition to other factors such as elevated plasma concentrations of the offending drug and viral infectious triggers are all thought to contribute to the immunopathogenesis of all types of SCAR’s ( 1, 5). Mortality rates of up to 67% in TEN, 40% in SJS and 10% in DRESS have been reported ( 4). Although these conditions are rare, they carry significant morbidity and mortality, particularly if the offending drug is not withdrawn ( 1). Medications are the most common cause of SCAR’s causing >85% of cases of SCAR’s in adults, of which beta-lactams are frequently implicated ( 2, 3). Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) is another delayed cutaneous exanthema which can be severe. Severe cutaneous adverse reactions (SCAR’s) are a heterogeneous group of delayed T cell mediated hypersensitivity reactions, which include Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS) and acute generalized exanthematous pustulosis (AGEP) ( 1). Based on our experience we provide clinicians with a practical algorithm for testing for cross-reactivity in non-SJS/TEN severe cutaneous adverse reactions.
#Allergy cross reactivity chart series
In our case series cross-reactivity or co-reactivity commonly occurred among the beta-lactam antibiotic class, however further research is required to investigate and understand patterns of cross-reactivity.

We assessed patients presenting with non-SJS/TEN severe cutaneous adverse reactions to a tertiary hospital drug allergy clinic. Historically, these reactions were considered to be specific to the inciting antibiotic and therefore likely to have minimal cross-reactivity. The underlying immunopathogenesis of these reactions is not completely understood but involves interactions between small molecule drugs, T cells and HLA molecules. 2Medical School, University of Western Australia, Nedlands, WA, AustraliaĬurrent understanding of cross-reactivity in severe cutaneous adverse reactions to beta-lactam antibiotics is limited, thereby making recommendations for future prescribing difficult.1Queen Elizabeth II Medical Centre, Department of Clinical Immunology, Sir Charles Gairdner Hospital, Pathwest, Nedlands, WA, Australia.Grace Thompson 1, Andrew McLean-Tooke 1 and Michaela Lucas 1,2*


 0 kommentar(er)
0 kommentar(er)
